
Overview
Phase 2 of Axia’s End-to-End Pilot was built on the success of Phase 1, which demonstrated the technical feasibility of applying RFID to various drug formulations and pharmaceutical packaging types in a laboratory setting. Phase 2 significantly expanded the scope to test the robustness and interoperability of RFID across the pharmaceutical supply chain, in alignment with GS1 standards and the U.S. FDA’s Drug Supply Chain Security Act (DSCSA) requirements.
For the first time, tests were conducted using four full pallets of pharmaceutical products—with varying formulations and packaging sizes—across diverse environments, including the Axia Lab (simulating the manufacturer and pharmacies) and Cencora’s Williamston, MI, Distribution Center. A pivotal component of the project was the development of the Axia Observer Platform, an IoT- based software solution designed to track materials across the supply chain using both RFID scans and Electronic Product Code Information Service (EPCIS) supply chain event data.
Key Highlights


100%
Traceability
achieved across the supply chain
6,920
Units Tracked
in operational environments
>0.3%
Human Errors
identified by RFID and corrected
Pilot Phase 2 Goal: Real-World Demonstration
To conduct a real-world demonstration and develop a cloud-based platform capable of collecting and transferring information captured by RFID readers at different locations to track pharmaceuticals through the supply chain.
Major Nodes of the Pilot Pharmaceutical Supply Chain

Phase 2 Participants
The following companies and organizations participated in Phase 2.
Timeline
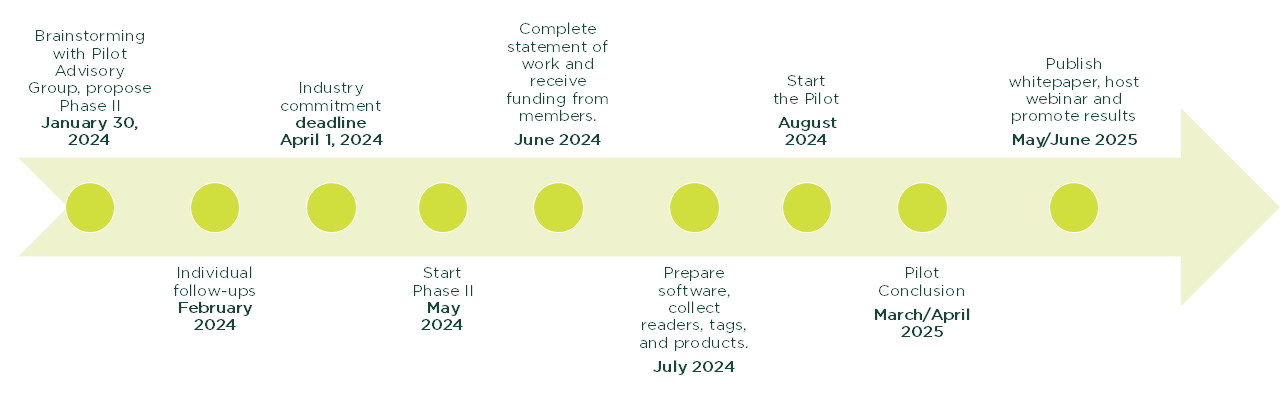
Deliverables
Validate RFID Technology to Provide Traceability: ACHIEVED
A total of 6,920 products were successfully tracked, achieving 100% traceability using RFID. Exceptions were automatically identified and corrected in real-time. The pilot demonstrated readiness for DSCSA-aligned digitization of the pharmaceutical supply chain at scale.
Develop Small-scale Data Capturing Platform: ACHIEVED
Axia’s Observer Platform was developed and tested as an outside centralized platform to provide oversight of the supply chain. The Axia Observer Platform provides: real-time monitoring, anomaly detection, master data integration, product flow integration and more.
Comprehensive Report: ACHIEVED
A whitepaper titled “Drug Supply Chain Security Act (DSCSA) and the Pharmaceutical Supply Chain” was published in May 2025. Axia was asked to present key findings at the RFID Journal Live on May 7 in Las Vegas. AIM (Association for Automatic Identification and Mobility) hosted a webinar on May 21 titled, “Can RFID Take Us Beyond DSCSA to Stop Counterfeits, Improve Safety and Streamline Pharma?” during which findings were also shared.
Want to learn more?
Where industry sees a challenge, we see opportunity. The Axia Institute partners with organizations and corporations worldwide to address the grand challenges that keep leaders and executives awake at night.
We collaborate to develop novel solutions that are getting the attention of industry leaders, government agencies and global organizations alike.
Learn about Joining Axia today.
Partnerships:
Collaborating for Success
Bridging the gap from theory to real-world application.
Education:
Developing Your Skills
Graduate studies, certificate programs and seminars in value chain creation and optimization.
Contact:
The Axia Institute
Have questions? Learn more about how you can get involved with The Axia Institute.







