
Goal Pilot Phase I
The primary goal of this project was to evaluate the efficiency of RFID technology as a transformative tool for achieving DSCSA compliance and optimizing pharmaceutical supply chain operations.
Pilot Advisory Group
The following companies and organizations were part of the Phase I Pilot Group and were instrumental in defining the real-world workflows and critical to the pilot.
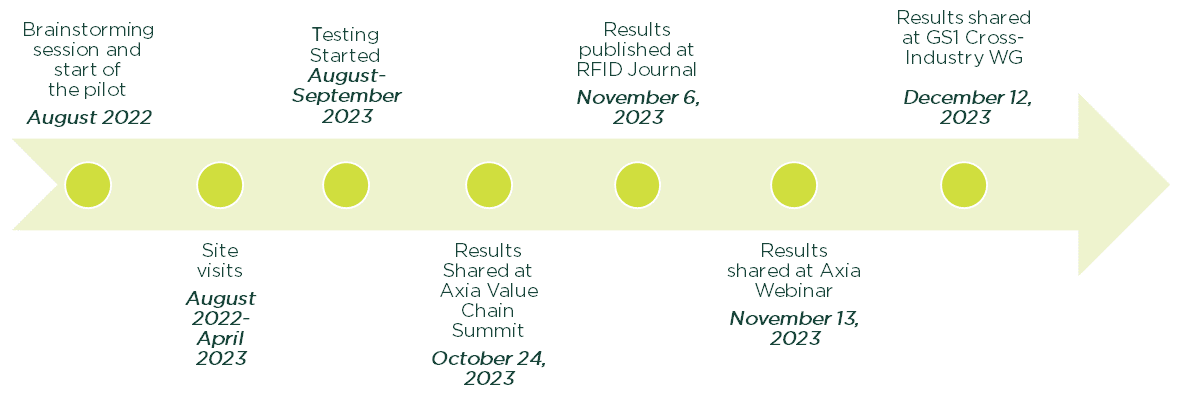
Timeline
Lab Scale Pilot

Step I: Igniting Industry-Academia Collaboration
A two-day immersive experience by various healthcare stakeholders included discussions on the implementation of DSCSA, interoperability, and RFID technology.
Step II: Real-World Workflows
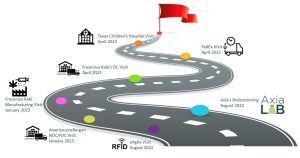
The advisory group conducted six site visits to learn each critical node in the pharmaceutical supply chain: a pharmaceutical manufacturer, distributor, transportation, hospital and a solutions provider.
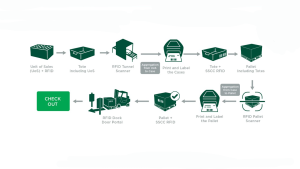
Step III: Building a Lab-Scale Pharma Supply Chain
Based on site visits, advice from our advisory group, the process flow was designed to mimick a real-world pharmaceutical supply chain.
In the Lab Pilot, three UHF RFID tags were used, all of similiar size, from three different manufacturers. The encoding scheme followed GS1’s serialization standard. Pharmaceutical products from three independent manufactuers were used. Two totes were created, one with more solid tablets than liquid vials and two, had more liquid vials than oral tablets. However, the study showed that the tag design plays more of a significant role in higher readability than tote formulation or read time.
By the Numbers
100%
Readability
at pallet level
In all tags and formulations in 15 sec.
99.67%
Highest Readability
at tote level
In all tags and formulations in 2.5 sec.
96.50%
Lowest Readability
at tote level
In all tags and formulations in 1.5 sec.
100
Tests
Each tag and formulation were tested 100 times.
Conclusions

RFID Technology Can Strengthen Pharma Supply Chain
1. RFID is a viable technology capable of strengthening the pharmaceutical supply chain to support ongoing efforts to improve patient safety.
2. This initiative has set the stage for more in-depth investigations into the cost-effectiveness and error-reduction benefits of RFID technology when compared to traditional barcoding methods.
3. We need to expand the scope of our testing with additional products to demonstrate the efficacy of this technology.
Deliverables
Formation of the “Pilot Advisory Group”: ACHIEVED
A diverse group of stakeholders from the healthcare industry came together as the “Pilot Advisory Group.” This collaborative effort provided invaluable guidance on the framework and logistics of the pilot initiative.
Site visits across the entire pharmaceutical supply chain: ACHIEVED
Visits to at least one site representing each crucial point within the pharmaceutical supply chain were conducted. These site visits offered direct exposure and knowledge acquisition, enabling the identification of potential vulnerabilities and risks within the supply chain. The insights gathered played a fundamental role in designing a process that could faithfully emulate real-world scenarios on a smaller scale within the laboratory.
Development of a benchmark laboratory testing framework: ACHIEVED
The project successfully developed a benchmark laboratory testing framework. This framework, which is the first one in the field, is not only essential for the next phase of the pilot but also serves as a valuable resource for future RFID use cases.
Creation of tailored software solutions: ACHIEVED
In collaborating with ACSIS, a customized software solution was developed for RFID labeling aggregation and the demonstration of traceability across critical reading points. This software can be further improved to provide an end-to-end data capture, compliant with DSCSA and it can be used for other applications for food traceability.
Partnerships:
Collaborating for Success
Bridging the gap from theory to real-world application.
Education:
Developing Your Skills
Graduate studies, certificate programs and seminars in value chain creation and optimization.
Contact:
The Axia Institute
Have questions? Learn more about how you can get involved with The Axia Institute.












