AXIA ARTICLE
April 18, 2024
The Urgent Need for Connected Labels in Ensuring Our Safety
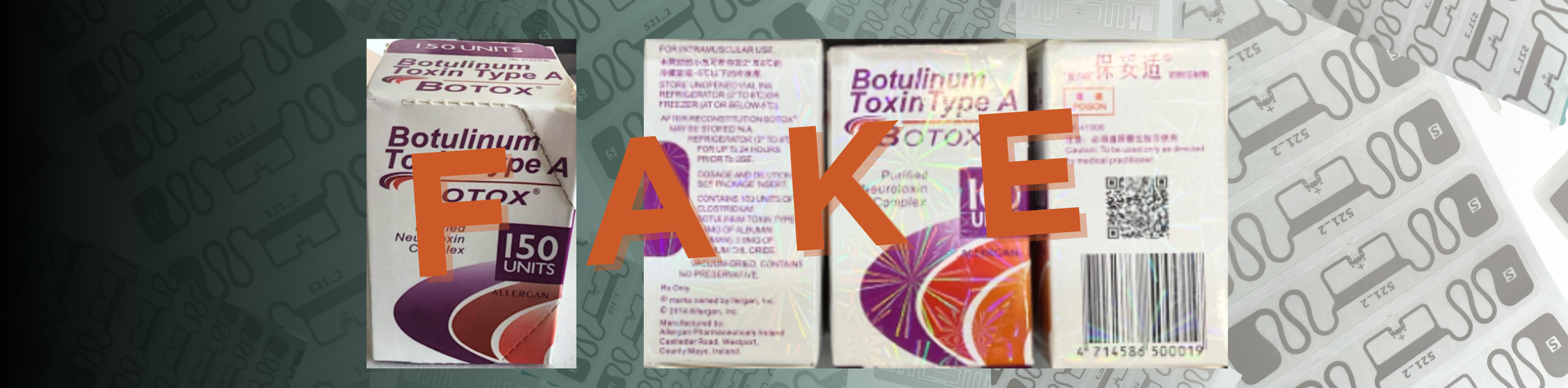
In light of recent events involving counterfeit Botox, the looming threat of fake drugs presents a grave danger to public health. With the FDA sounding the alarm on unsafe Botox, with replica boxes surfacing across several states, it’s paramount to confront the broader issue of counterfeit pharmaceuticals head-on and seek innovative strategies to uphold medication safety.
Counterfeit medications not only imperil patient well-being but also erode trust in our healthcare infrastructure. The reported symptoms from those exposed to counterfeit Botox underscore the severe repercussions of fake drugs, spanning from impaired vision to respiratory distress. These incidents serve as a stark reminder of the pressing need for heightened measures against drug counterfeiting.
One promising avenue lies in the widespread adoption of Radio Frequency Identification (RFID) and Near Field Communication (NFC)-based validated, connected labels for pharmaceuticals.

By integrating these advanced technologies into drug packaging, we can establish a robust system for verifying medication authenticity throughout the supply chain. RFID/NFC-connected labels facilitate real-time tracking and authentication, empowering healthcare professionals and consumers to validate drug legitimacy before use.
One promising avenue lies in the widespread adoption of Radio Frequency Identification (RFID) and Near Field Communication (NFC)-based validated, connected labels for pharmaceuticals. By integrating these advanced technologies into drug packaging, we can establish a robust system for verifying medication authenticity throughout the supply chain. RFID/NFC-connected labels facilitate real-time tracking and authentication, empowering healthcare professionals and consumers to validate drug legitimacy before use.

Swaminathan “Swami” Subramanian is the director of business development at the Axia Institute, where he cultivates corporate partnerships and supports research efforts, innovation, and the growth of the institute’s research portfolio. Swami is leading the Patient-Centric Drug Information Distribution Program with drug manufacturers, distributors, pharmacies, and solution providers and can be reached at sswami@msu.edu.
Want to become part of the e-Leaflet Consortium?
Where industry sees a challenge, we see opportunity. The Axia Institute partners with organizations and corporations worldwide to address the grand challenges that keep leaders and executives awake at night.
We collaborate to develop novel solutions that are getting the attention of industry leaders, government agencies and global organizations alike.
Learn about Joining Axia today.
Partnerships:
Collaborating for Success
Bridging the gap from theory to real-world application.
Education:
Developing Your Skills
Graduate studies, certificate programs and seminars in value chain creation and optimization.
Contact:
The Axia Institute
Have questions? Learn more about how you can get involved with The Axia Institute.
